Format follows function
The human genome encodes for more than 500 known kinases (the kinome). Many of these kinases maintain essential cellular functions and differ greatly in both function and expression profile in response to external stimuli. Mutations and overexpression of kinases leads to aberrant functioning and is associated with the initiation, progression, and recurrence of cancer.
Targeting kinases associated with cancer development and progression has proven to be an effective treatment modality. To-date more than 70 kinase inhibitors have been approved for multiple oncology indications.
In the design of novel therapeutics, it is essential to consider the function of the targeting kinase and its expression profile in the human body.
At Crossfire we have developed a proprietary platform which allows us to approach kinases from three different angles depending on whether we want to:
- Target wild type kinases, or also/only specific mutant variants
- Inhibit or degrade the kinase of interest
- Deliver the drug in every cell, or antibody-mediated delivery to predefined cells only
Based on scientific background, a deep understanding of the biology and empirical testing we select the most suitable platform for the design of kinase targeting drugs with an optimal therapeutic effect.

- (Mutant) kinases with aberrant signaling and (tumor-)restricted expression will benefit from EPriL inhibitors.
- (Mutant) kinases with aberrant signaling and scaffolding and (tumor-)restricted expression will benefit from EPriL degraders.
- Kinases with a broad expression profile will benefit from an antibody targeted DAC approach.
EPriL inhibitors are being developed to target (mutant) kinases with aberrant signaling and (tumor) restricted expression.
Crossfire’s Energetically Privileged Ligands (EPriLs) are non-covalently binding, but designed to have a long, covalent-like, residence time on target kinases. EPriL-based Inhibitors show prolonged signaling inhibition compared to other non-covalent inhibitors in clinical development. By targeting both wild-type and mutated forms of a kinase, our inhibitors reduce the risk of tumor escape through acquired mutations. Furthermore, EPriL-based inhibitors maintain high selectivity over other kinases of the kinome (off-target kinases) reducing the risk of toxicity in non-cancerous cells and tissues.
Our BTK inhibitor (CFON-026) is our most advanced internal program, with clinical trials planned in in the second half of 2025. CFON-026 is being developed as a potential best-in class non-covalent inhibitor, targeting both wild type BTK and all clinically relevant BTK mutant variants.

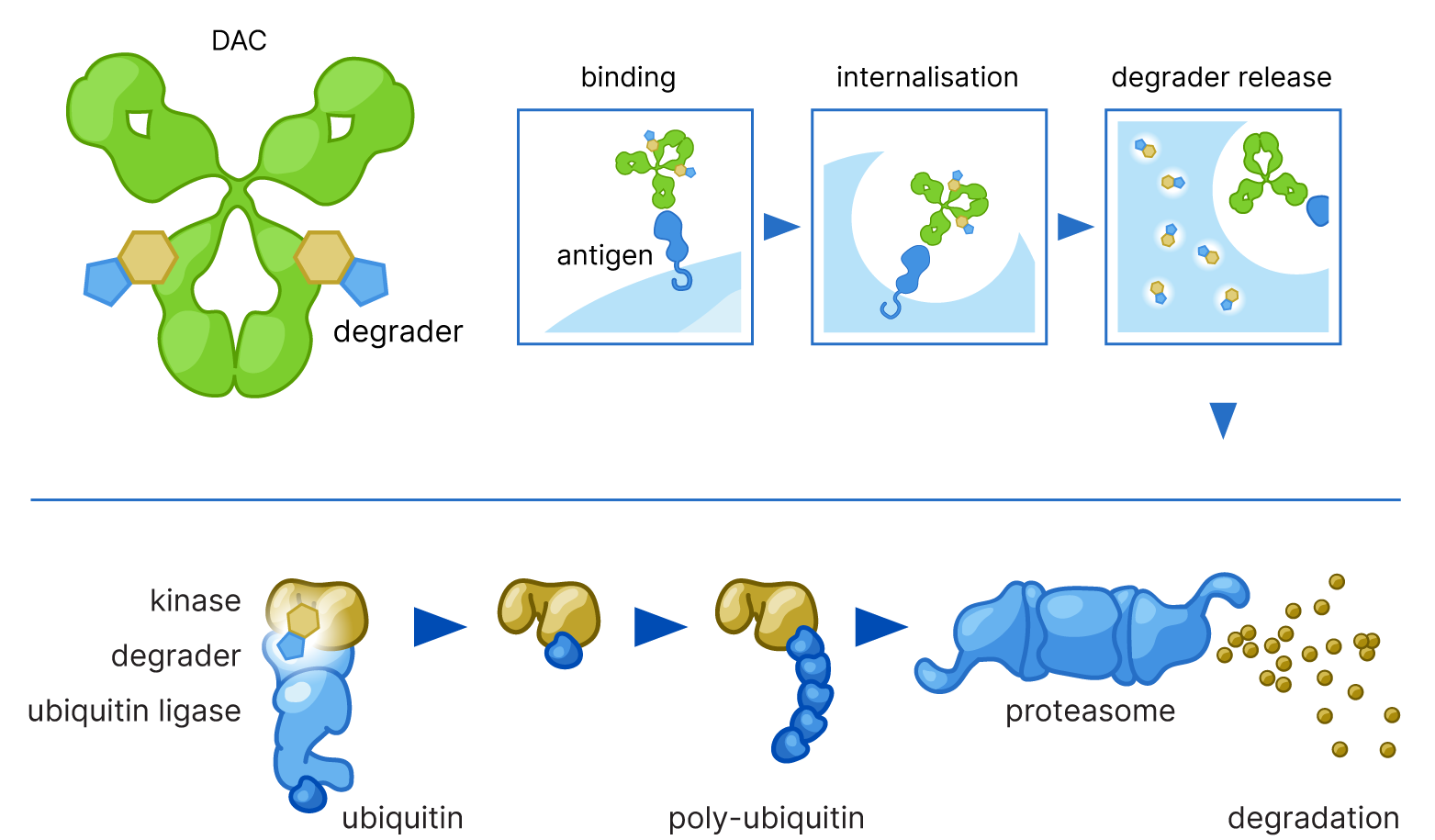
(Mutant) kinases where besides signaling also aberrant scaffolding plays an important role and expression is (tumor) restricted, Crossfire has developed EPriL-based degraders. These work via heterobifunctional targeting of a kinase and an E3 ubiquitin ligase directing the target kinase to the proteasome of the cell for degradation. Selective targeting of both wild-type and mutated kinases reduces the risk of tumor escape. The combination of high selectivity and long target residence time allows for very efficient degradation.

For kinases with a broad expression profile Crossfire is developing a so-called Degrader Antibody Conjugate (DAC) approach. By linking a highly potent EPriL-based degrader to a monoclonal antibody specific to a tumor associated antigen, targeted delivery of the degrader is achieved. This improves the selective killing of tumor cells, potentially increasing efficacy and reducing systemic toxicity by leaving healthy cells unharmed.